RadChemist
Plastic
- Joined
- Jun 6, 2018
Hi everyone,
This is my first post here.
I am a Ph.D. student doing research in the field of radiochemistry. One of my projects involves radionuclide production through proton bombardment of enriched target materials in a cyclotron. A key component of my research is designing robust targets that are capable of withstanding high temperatures during irradiation.
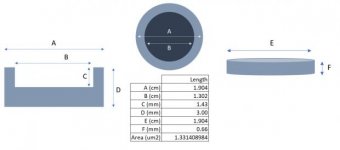
Our current design features an aluminum target casing filled with layers of compressed target material, and a thin aluminum lid is fastened to the top with an epoxy adhesive.
I would like to move away from using any type of chemical adhesive and instead rely on some type of mechanical attachment (the simpler the better) since we've had contamination problems with epoxy in the past.
I've included three attachments of the design with dimensions.
Any help will be greatly appreciated!
This is my first post here.
I am a Ph.D. student doing research in the field of radiochemistry. One of my projects involves radionuclide production through proton bombardment of enriched target materials in a cyclotron. A key component of my research is designing robust targets that are capable of withstanding high temperatures during irradiation.
Our current design features an aluminum target casing filled with layers of compressed target material, and a thin aluminum lid is fastened to the top with an epoxy adhesive.
I would like to move away from using any type of chemical adhesive and instead rely on some type of mechanical attachment (the simpler the better) since we've had contamination problems with epoxy in the past.
I've included three attachments of the design with dimensions.
Any help will be greatly appreciated!