can anybody point me to some info on activating metal (and glass) surfaces?
i see virtually nothing online, this is all i found:
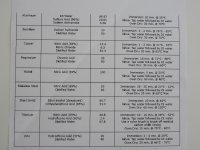
besides remembering don gelbarts video:
YouTube
scrubbing powder seems not to work (i tried three brands) and he doesns mention sanding, only sand blasting. what might be the difference?
i see virtually nothing online, this is all i found:

besides remembering don gelbarts video:
YouTube
scrubbing powder seems not to work (i tried three brands) and he doesns mention sanding, only sand blasting. what might be the difference?

